
A. Prof. Suwarno from INSTITUT TEKNOLOGI SEPULUH NOPEMBER, Indonesia visited Institute for Energy Technology, Norway, from 1 July 2018 until 1 October 2018 accomplishing a 3-months secondment.
Prof. Suwarno worked on the WP1:
Development and characterisation of advanced MH materials for hydrogen storage and compression.
In particular, the work was focused on two aspects.
- Studies of the Pressure-Composition-Temperature diagrams in a multicomponent Laves-type Ti, Zr, La, Ni, Mn, V, Fe alloys with a variable ratio between A and B components. The goal was to establish an interrelation between the content of B (Ni+Mn+V+Fe) in a group of the alloys with B changing as AB90; AB1.95; AB2.00; AB2.05.
- Studies of the binary systems Y-H and RE-H (RE=Dy, Ho, Er) using vacuum Thermal Desorption Spectroscopy.
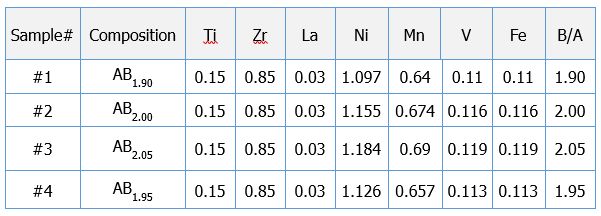
Compositions of the alloys are listed in the Table 1.
Table 1. Studied alloys.
A typical set of isotherms collected for the AB2.00 alloy are shown in the Figure 2.
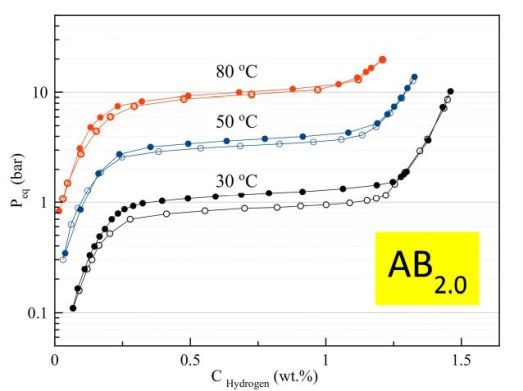
Figure 2. PCT isotherms of hydrogen absorption and desorption measured for the AB2.00 alloy at 30, 50 and 80 °C.
These studies showed a regular trend in changes of the thermodynamic stability of the formed hydrides. This is clearly seen from the data listed in Table 2.
Figure 3. Typical changes in the temperature dependence of equilibrium pressured of hydrogen absorption and desorption on one of the studied systems. A rapid decrease in hysteresis between pressures of Habs and Hdes takes place with temperature increasing to 80 °C.
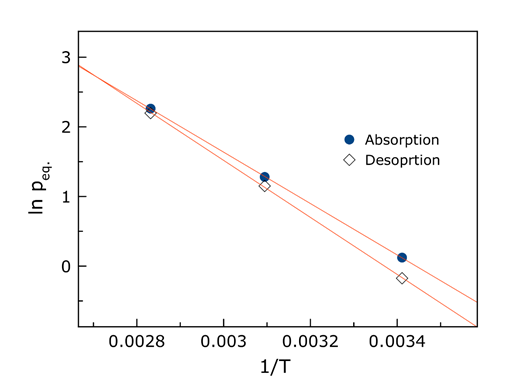
Table 2. Thermodynamic characteristics of the studied hydrides.
| Alloy | deltaH (kJ/mol) | deltaS (J/mol K) | |
| AB1.9 | Abs | 33.7±0.4 | 107.4±1.1 |
| Des | 35.2±0.4 | 111.4±1.1 | |
| AB1.95 | Abs | 32.4±0.9 | 105.5±3.0 |
| Des | 34.7±0.1 | 111.5±0.2 | |
| AB2.0 | Abs | 30.7±0.2 | 105.6±0.5 |
| Des | 34.1±0.5 | 114.7±1.5 |
Accurate evaluation of the collected data will be performed using a thermodynamic model recently developed by a project participant Dr. Michael Lototskyy at the University of the Western Cape. This has been discussed and agreed upon in a HYDRIDE4MOBILITY project meeting at IFE as Dr. Lototskyy stayed at IFE at the same time with Prof. Suwarno.
In the second part of the work related to the project Prof. Suwarno studied by Thermal Desorption Spectroscopy properties of binary rare earth metals, Dy, Ho, Er and Y.
One typical set of the collected data is shown in Figure 4 and presents the results for the Y-H binary system.
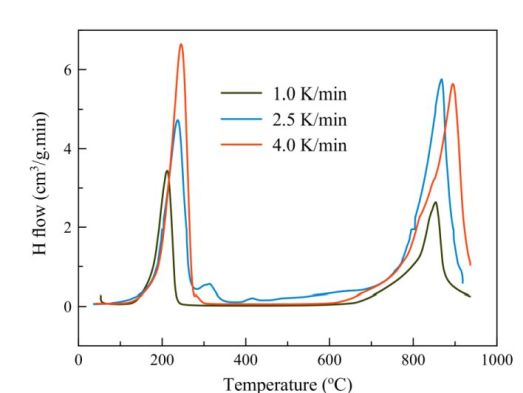
Figure 4. Vacuum thermal desorption spectra measured for YH3 hydride at heating rates 1; 2.5 and 4.0 K/min.
Analysis of the data shows that thermal stability of the studied hydrides changes in a raw
ErH2<DyH2<HoH2<YH2.
The transformation of ReH2àRe accompanied by hydrogen desorption follows the Avraami-Erofeev model of 3-dimensional nuclei growth.
The activation energy is ranging from 170 kJ/mol for ErH2 to ~250 kJ/mol for DyH2 and HoH2.
The results of the work will be published in two publications and will be presented in a poster presentation by Prof. Suwarno at International Symposium on Metal Hydrogen Systems in China MH2018 (28 October – 2 November 2018).
Project manager
Prof. V.A. Yartys
Prof. Suwarno
2 October 2018

